Government recommends prescription for dermal fillers
Dermal fillers should, like botox, require a prescription and all injectors should be required to pass a qualification and join a register, according to the report of the Government's Keogh Review, announced today.
The Government review into the safety of cosmetic surgery and non-surgical procedures such as Botox and fillers, has today put its recommendations to UK health ministers.
Many in the beauty industry feared the report would recommend that only medical professionals should be allowed to carry out dermal filler procedures, which has not been the case. However, the report is likely to have significant implications for salons and clinics that do choose to carry out such procedures.
Alongside classifying dermal fillers as prescription only, the report’s recommendations include ensuring all practitioners are properly qualified for the procedures they offer, an advertising code of conduct, and an ombudsman to oversee all private health care including cosmetic procedures to assist consumers that have received poor treatment.
Crisis waiting to happen
The Review labeled the dermal fillers market, which is currently entirely unregulated, as a “crisis waiting to happen”.
Babtac chair Carolyne Cross has welcomed the outcome of the report, saying, “We believe that there will be some significant changes in terms of regulations and qualifications, and as an organisation we have been driving for improved standards of training and regulation of our own industry providers."
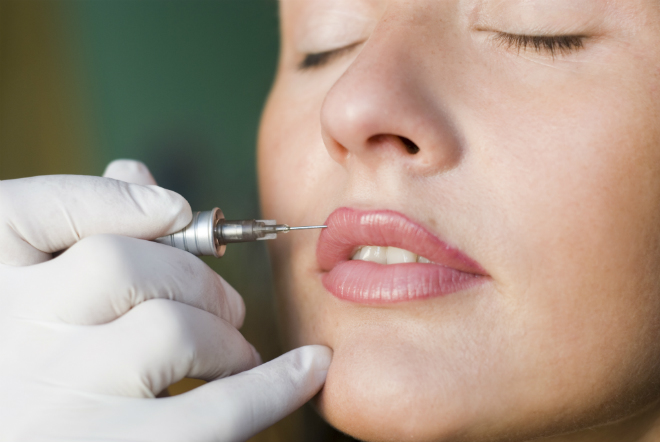
She added that it was likely therapists wishing to offer such treatments would have to under go additional training to become compliant, but the saftey guarantee to clients would be worth the additional expenditure.
Dermatologist Dr Nick Lowe told Professional Beauty that he was very much in favour of the move to make dermal fillers prescription only, saying that it would be an opportunity to give some excellence in training to the injectors. “It also will mean that the fillers themselves will have to be more carefully scrutinised,” he added.
Allergan, which manufactures Botox and also popular dermal fillers such as Juvéderm, also welcomed the review's recommendations to improve safety but suggested that some of the recommendations could add unnecessary work for practitioners.
Regarding the implementation of a new standardised qualification for anyone wishing to inject fillers, Allergan said in a statement, "Any legislation change of this type will require significant time and could also mean that medical aesthetic nurses, who are generally already highly skilled and experienced, will need to re-train in order to obtain a prescribing licence."
The cosmetic procedures sector was estimated to be worth £2.3 billion a year in 2010, and is predicted to grow to £3.6 billion by 2015.

