Originally posted https://aestheticmed.co.uk/ukhsa-issues-warning-over-botulism
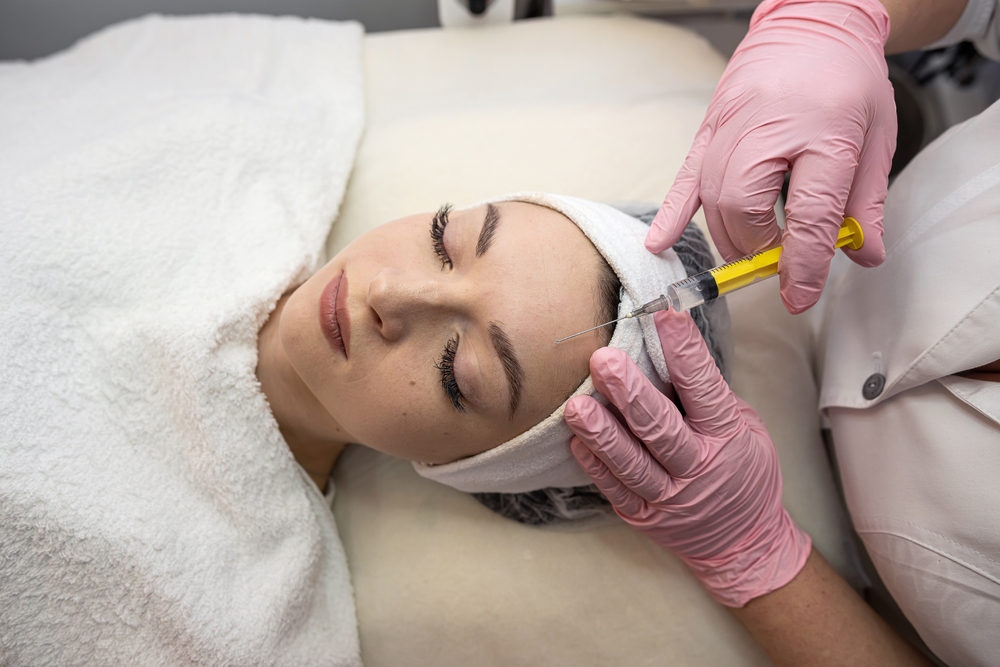
Forty-one cases of botulism have now been recorded in England following the suspected use of unlicensed 'Botox-like' products in cosmetic procedures, the UK Health Security Agency (UKHSA) said in an updated statement on 6 August 2025.
The agency is warning people to be aware of the signs and symptoms of botulism after a group of individuals presented to the National Health Service (NHS) after experiencing adverse reactions following botulinum toxin injections.
The cases have been reported in the North East, East Midlands, East of England, North West and Yorkshire and Humber. In the UKHSA's previous statement released on 18 July the reported amount of cases was 38.
The number of clinically confirmed cases of iatrogenic botulism reported between 4 June and 6 August 2025 is now 41.
UKHSA say that investigations are ongoing, but evidence so far suggests the use of an unlicensed 'Botox-like' or fake product. It is understood that those practitioners involved in this latest incident have ceased the procedure and are cooperating with the ongoing investigation.
Reactions have included:
-
difficulty swallowing
-
slurred speech
-
breathing difficulty requiring respiratory support.
UKHSA has issued national advice to clinicians to ensure that they look out for botulism in people who may have had a recent aesthetic procedure to provide them with appropriate treatment, which includes administering anti-toxin.
UKHSA is also advising people to take precautions when seeking aesthetic procedures, including checking if the product being used is licensed.
"We are working closely with our partners to reduce the public health risk and would advise people to make sure they take precautions when seeking aesthetic procedures. Botulism related to aesthetic procedures is rare, but it can be serious," said Dr Gauri Godbole, consultant medical microbiologist at UKHSA.
"It is caused by toxins produced by the bacterium Clostridium botulinum. These toxins (but not the bacteria) are the active ingredient in ‘Botox’ and similar products."
Dr Godbole says that symptoms of botulism can take up to 4 weeks to develop and advises that patients who have had a recent botulinum toxin treatment and are having symptoms such as difficulty swallowing or breathing, contact NHS 111 for further advice and seek treatment.
"When these procedures go wrong, there is a risk of serious infections and permanent scarring, which is why only registered professionals like a doctor, a nurse or pharmacy prescriber should be prescribing these treatments," added professor Meghana Pandit, co-national medical Director, secondary care at NHS England.
"Public safety is a top priority for the MHRA. Botulinum toxin is a prescription-only medicine and should only be sold or supplied in accordance with a prescription given by an appropriate practitioner such as a doctor or other qualified healthcare professional," said Dr Alison Cave, MHRA Chief Safety Officer.
"Buying botulinum toxin in any other circumstances significantly increases the risk of getting a product which is either falsified or not licensed for use in the UK. This means that there are no safeguards to ensure products meet the MHRA’s standards for quality and safety. As such, they can endanger the health of the people who take them."
"Our Criminal Enforcement Unit works hard to identify those involved in the illegal trade in medicines and takes robust enforcement action where necessary. This can include criminal prosecution."